Vaccination is one of the greatest health
success stories of the last two centuries,
eradicating smallpox and nearly eliminating
diseases like polio, worldwide.1
In order to fulfil the unmet medical need, vaccine
development requires complex technical expertise
and sophisticated scale-up processes.


An idea is born!
Through intensive study of a virus, scientists attempt to understand the structure of this bug.
This allows researchers to mimic the virus and identify ways to equip the human body with immunity to fight the virus. 2

A rigorous process
With vaccine discovery, quality, efficacy, and safety are paramount. For this reason, before a vaccine
is licensed and brought to market
it undergoes a long and rigorous period of research followed by many years of testing and continual surveillance to ensure that all of these conditions are met.4
THE REGULATORY PROCESS

If the quality, efficacy and safety meets the regulatory requirements and the benefit-risk is established with the authorities, the vaccine should secure approval.
When we turn on a tap, we expect clean water to come rushing out on demand. However, we’re often not aware of the amount of work and effort that has gone into bringing the water to our homes.
The same can be said for vaccines
– there is an incredible amount
of time, care and effort which
goes into the development
of each and every vaccine.
Hidden mastery
and unseen effort…

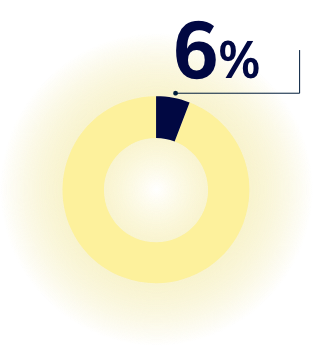
Did You Know?
In a non-pandemic situation, when scientists search for a vaccine, vaccine development can take up to 20 years. Vaccine discovery is a challenging science and only few vaccines (approx. 6%) make it from early development to market. 5
Only if the quality, efficacy and safety meets the regulatory requirements and the benefit-risk is established with the authorities, the vaccine should secure approval.

Considered the father of modern vaccines, MSD was privileged to have worked with
Dr. Maurice Hilleman, a man whose work is estimated to have saved more lives worldwide than any other medical scientist in the 20th century. 7
During the course of his remarkable career, including 25 years of work with MSD, Dr. Hilleman helped develop more than 40 vaccines for human and animal health, including many against childhood illnesses that were once common throughout the world. 7
During the course of his remarkable career, including 25 years of work with MSD, Dr. Hilleman helped develop more than 40 vaccines for human and animal health, including many against childhood illnesses that were once common throughout the world. 7